Solid State techniques were applied to investigate API raw material attributes and their impact on the final dosage formulations between two API vendors.
XRD, DSC, TGA | No polymorphic changes but crystal morphological variations could be observed between APIs |
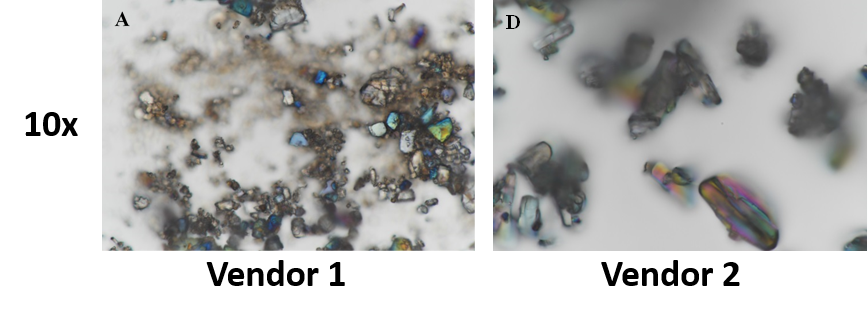
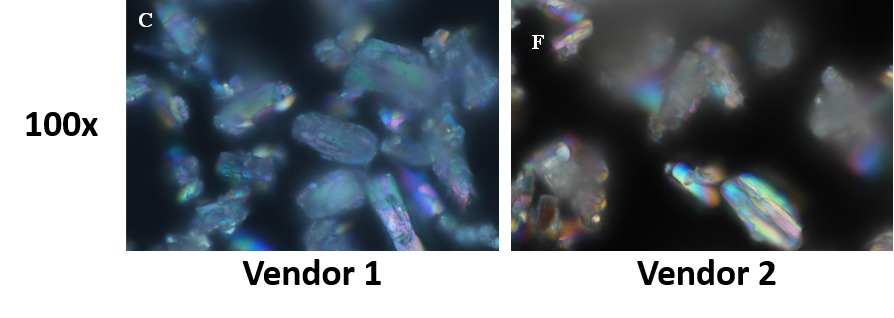
SEM | Sun API is morphologically uniform, however, Symed API has particles of different shapes and sizes |
Raman Peak Ratio | Variations in molecular level properties observed and attributed to different crystallinity |
NIR | NIR indicate that the tablet properties arrived in RLD using Symed API cannot be achieved using Sun API. Process change required. |
Conclusions And Recommendations
- API chemical properties/polymorph are found to remain similar
- Physical properties like morphology, particle level disorder and shape were found to significantly differ.
- Process adjustment can be carried out to circumvent the variations observed in dissolution
Raw material qualification system ensuring consistency of material from lot to lot/vendor to vendor, sufficing Life Cycle management requirements is established
Muthudoss et al., A. Vendor qualification: Utilization of solid state characterization “Toolbox” to assess material variability for active pharmaceutical ingredient. Journal of Applied Pharmaceutical Science. 2019 Sep 1;9(9):001-9. https://japsonline.com/abstract.php?article_id=2972#:~:text=10.7324/JAPS.2019.90901
