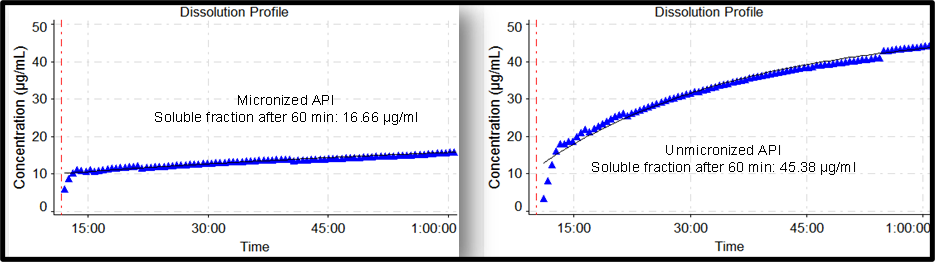

Problem Statement
- Micronized API demonstrated slower dissolution than the Unmicronized API
- No changes in polymorphism or particle size is envisaged
Business Need
- To study the effect of agglomeration on micronized model active pharmaceutical ingredient (API) and to unravel the influence of API agglomerate strength on dissolution and permeation
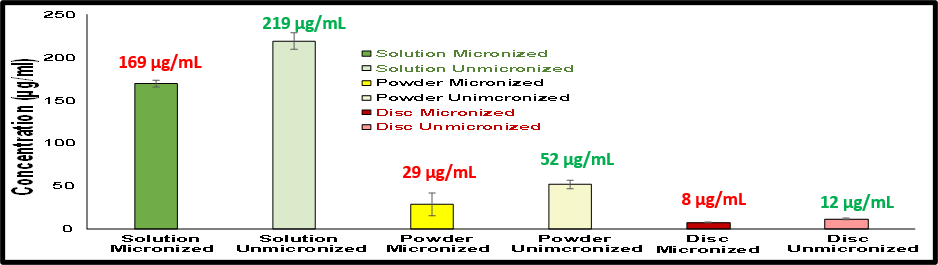
Comparison of Intrinsic Dissolution from Solution vs. Powder vs. Disc
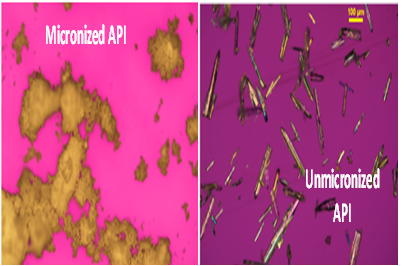
PLM: Seeing is Believing
Intrinsic Dissolution-Mimicking Various Formulation
- Solution: 10 mg of API is Predissolved in DMSO (resembling solution/injection)
- Powder: 10 mg dose of API weighed & added (Capsule)
- Compacted Disc: 10 mg of powder is weighed and compressed to pellet (resembling tablet)
Discussion
Results indicated that API predissolved in DMSO had higher drug release than powder and compressed disc
Microscopy suggests agglomeration to be contributing reason for differences in intrinsic dissolution rates
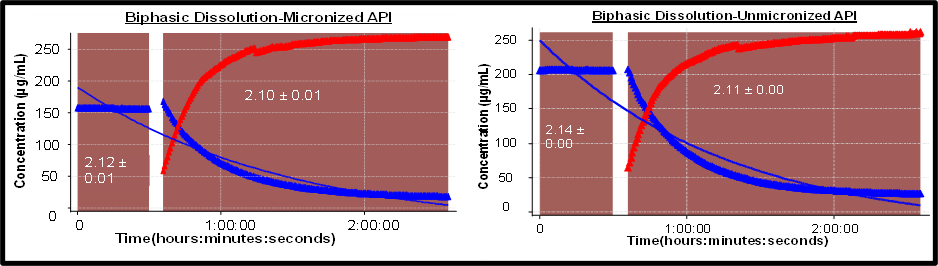
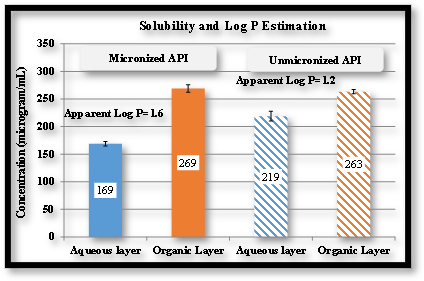
Biphasic Dissolution: Pre-dissolved drug was injected in aqueous layer, partitioning in octanol measured
- Blue Curve Indicates dissolution, red indicates partition.
Predicting the impact on Permeation
- Predissolved API in DMSO was used as the model
- Results indicated that if the API is available dissolved then partitioning is not a concern
- API demonstrates dissolution-limited permeation
Novel method to monitor impact of Micronization and agglomeration on Final product is Demonstrated
Muthudoss et al., Micronization and agglomeration: understanding the impact of API particle properties on dissolution and permeability using solid state and biopharmaceutical “toolbox”. Journal of Pharmaceutical Innovation. 2021 Mar;16(1):136-51. https://doi.org/10.1007/s12247-019-09424-1
